In a world increasingly aware of the harmful effects of ultraviolet (UV) radiation, accurate measurement of UV light is paramount for various fields including health, safety, and technology. From assessing the sun’s impact on skin to ensuring effective sterilization in medical settings, understanding and quantifying UV exposure is essential. But how exactly do we measure UV light?
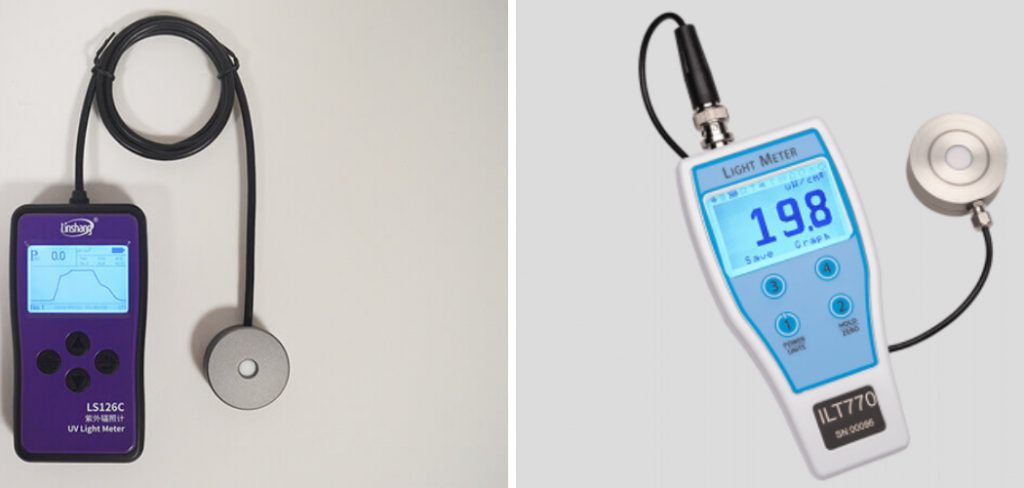
This comprehensive guide delves into the intricacies of UV measurement techniques, exploring both conventional methods and modern advancements. From basic handheld UV meters to sophisticated spectrophotometers, the tools available for UV measurement cater to diverse needs and applications. We’ll unravel the science behind UV measurement, discussing key parameters such as intensity, wavelength, and exposure duration.
Moreover, we’ll explore emerging technologies like UV imaging and wearable sensors, revolutionizing how to measure uv light in real-time. Whether you’re a scientist, engineer, or simply a health-conscious individual, mastering the art of measuring UV light is indispensable in safeguarding yourself and others from its potential harms.
Definition of UV Light
Ultraviolet (UV) light is a type of electromagnetic radiation that falls between visible light and X-rays on the electromagnetic spectrum. It has wavelengths ranging from about 10 nanometers to 400 nanometers, shorter than that of visible light but longer than X-rays.
UV light is not visible to the human eye and is classified into three main types based on its wavelength: UVA (315-400 nm), which reaches the earth’s surface and is associated with skin aging; UVB (280-315 nm), which is partially absorbed by the ozone layer but can still cause skin burns; and UVC (100-280 nm), which is almost entirely absorbed by the earth’s atmosphere and has potent germicidal properties. Understanding these classifications is crucial in measuring UV light and managing its effects on health and the environment.
Importance of Measuring UV Light
Measuring UV light is not just a matter of scientific interest; it’s a crucial step in protecting human health and safety. Overexposure to UV radiation can lead to serious health issues, including skin cancer, cataracts, and immune system suppression.
For industries and services relying on UV light, such as water purification and medical sterilization, precise measurement ensures that processes are effective without being harmful. In the agricultural sector, understanding UV exposure can improve crop yield and quality.

Additionally, in fields like art preservation and forensic analysis, accurately gauging UV light helps prevent degradation and provides crucial information. Hence, the ability to measure UV radiation accurately is vital across various sectors, safeguarding against its harmful effects while harnessing its benefits.
Properties of UV Light
UV light possesses unique properties that distinguish it from other forms of electromagnetic radiation, making its measurement both challenging and essential. One of the fundamental properties of UV light is its ability to cause chemical reactions that can lead to changes or damage in biological tissues, an effect not seen with visible light.
This property is what makes UV light both useful, for processes like sterilization, and harmful, contributing to skin cancer and other health issues. Another critical property is its varying wavelength categories (UVA, UVB, and UVC), each with different effects on the environment and living organisms. This variance in wavelengths necessitates specialized equipment and techniques for accurate measurement.
Furthermore, UV light’s energy and intensity can vary depending on factors like time of day, season, and altitude, complicating the process of obtaining precise readings. Understanding these properties is vital for effectively measuring UV light and mitigating its adverse effects while maximizing its beneficial uses.
Types of UV Light
UV light is categorized into three distinct types, each differentiated by its specific wavelength range, which, in turn, influences its interaction with the environment and living organisms:
- UVA (315-400 nm): This is the longest wavelength of the UV spectrum and constitutes the majority of UV radiation reaching the Earth’s surface. UVA can penetrate deeper into the skin layers, leading to skin aging and long-term damage. Unlike UVB, UVA is present with relatively stable intensity throughout all hours of daylight and during the entire year.
- UVB (280-315 nm): UVB has a medium range wavelength and is biologically active but is mostly absorbed by the ozone layer, with only a small portion reaching the Earth’s surface. It is responsible for sunburn and plays a critical role in causing skin cancers. UVB intensity varies depending on the time of day and season, with its peak during summer months.
- UVC (100-280 nm): The shortest wavelength of the UV spectrum, UVC, is completely absorbed by the Earth’s atmosphere and does not reach the surface. It possesses potent germicidal properties and is used extensively in sterilization processes in medical and water treatment facilities.

Understanding these types and their distinct characteristics is crucial for the effective measurement and application of UV light, as well as for taking protective measures against its potential harmful effects.
Sources of UV Light
UV light is emitted from a variety of natural and artificial sources, each contributing differently to the levels of UV radiation that humans and the environment are exposed to.
The primary natural source of UV light is the sun, which emits UV radiation as part of its broad spectrum of light. This sunlight is the most significant source of UV radiation for most people and environments on Earth, influencing ecosystems, weather patterns, and human health.
In addition to natural sunlight, there are several artificial sources of UV light, developed for specific purposes across industries and research. These include:
- UV Lamps: Widely used in industrial processes, medical treatments, and water purification systems, these lamps are designed to produce specific wavelengths of UV light, particularly UVC, for sterilization and disinfection purposes.
- Tanning Beds: Tanning beds and booths are significant artificial sources of UV radiation, primarily emitting UVA, with some emitting UVB. These are used for cosmetic tanning but pose health risks similar to natural sun exposure.
- Black Lights: These are special lamps that emit long-wave UVA radiation and are commonly used for artistic and decorative effects, as well as in forensic analysis and insect control devices.
- Mercury-Vapor Lamps: These lamps are used in street lighting and to promote plant growth in greenhouses. They emit UV radiation as part of their light, which is generally filtered out but can contribute to UV exposure in certain circumstances.
- Halogen, Fluorescent, and LED Lights: While primarily used for illumination, these common lighting technologies can emit small amounts of UV radiation, especially if their protective coatings are damaged or removed.
Understanding these sources and their emission patterns is crucial for effective UV light management, ensuring the benefits of UV light are harnessed while minimizing its harmful effects on human health and the environment.
10 Methods How to Measure Uv Light
1. UV Photometry:
UV photometry involves using photometric techniques to measure the intensity of UV light. This method relies on photodetectors that respond to UV radiation by producing an electrical signal proportional to the light intensity.
UV photometers typically use filters to isolate specific UV wavelengths for measurement. The output of the photodetector is then calibrated against known standards to quantify the UV intensity in units such as microwatts per square centimeter (µW/cm²).
2. UV Spectrophotometry:
UV spectrophotometry is a technique used to measure the absorption or transmission of UV light by a sample across a range of wavelengths. This method employs a UV spectrophotometer, which consists of a UV light source, monochromator, sample holder, and photodetector.
The monochromator selectively filters the UV light to a specific wavelength, which passes through the sample. The photodetector measures the intensity of the transmitted light, allowing for the determination of the sample’s absorbance or transmittance at that wavelength. UV spectrophotometry is commonly used in analytical chemistry for quantitative analysis of UV-absorbing compounds.
3. UV Radiometry:
UV radiometry involves the measurement of radiant flux or irradiance of UV light. Radiant flux refers to the total power of UV radiation emitted from a source, usually expressed in watts (W).
Irradiance, on the other hand, refers to the power of UV radiation incident on a surface per unit area, typically expressed in watts per square meter (W/m²). UV radiometers are instruments used to measure radiant flux or irradiance of UV light. These instruments may utilize photodetectors, such as photodiodes or photovoltaic cells, calibrated to respond specifically to UV radiation.
4.UV Dosimetry:
UV dosimetry involves quantifying the UV dose received by a surface or organism over a specific period. UV dosimeters are devices designed to measure cumulative UV exposure, taking into account both intensity and duration of exposure.

Dosimeters may employ chemical indicators that undergo a measurable change upon UV exposure, such as colorimetric or fluorescent dyes. Other types of dosimeters include electronic devices capable of integrating UV irradiance over time. UV dosimetry is essential for assessing UV exposure in applications like phototherapy, UV disinfection, and occupational safety.
5. UV Imaging:
UV imaging refers to the visualization of UV light emitted, transmitted, or reflected by an object or scene. UV cameras or imaging systems are equipped with sensors sensitive to UV wavelengths, allowing for the capture of UV images.
UV imaging can reveal details that may not be visible with visible light or infrared imaging, making it useful for various applications such as forensics, art authentication, and medical diagnostics. UV imaging systems may utilize CCD or CMOS sensors, often with specialized optics and filters to enhance sensitivity to UV light.
6. UV Microscopy:
UV microscopy involves examining samples under UV illumination to observe details not visible under visible light microscopy. UV microscopes are equipped with UV light sources and optics optimized for UV wavelengths. UV microscopy is particularly useful for analyzing fluorescent compounds, as many substances exhibit fluorescence when exposed to UV light.
Applications of UV microscopy include studying biological samples, analyzing materials for fluorescence properties, and investigating semiconductor devices.
7. UV Fluorescence Spectroscopy:
UV fluorescence spectroscopy is a technique used to analyze the fluorescence emitted by a sample upon excitation with UV light. This method exploits the unique fluorescence properties of certain compounds, which absorb UV radiation and re-emit it at longer wavelengths.

UV fluorescence spectrometers use a UV light source to excite the sample and a detector to measure the emitted fluorescence. By analyzing the intensity and spectrum of the fluorescence, valuable information about the sample’s composition and structure can be obtained. UV fluorescence spectroscopy finds applications in biochemical analysis, environmental monitoring, and materials science.
8. Actinic Radiation Measurement:
Actinic radiation refers to UV radiation capable of causing photochemical reactions in substances or organisms. Actinic radiation measurement involves quantifying the UV radiation that is biologically active or relevant to specific chemical processes.
Instruments used for actinic radiation measurement may include broadband radiometers calibrated to measure UV radiation within biologically significant wavelength ranges. Actinic radiation measurement is important for understanding the potential impact of UV radiation on living organisms, as well as for assessing the effectiveness of UV-based processes such as phototherapy and UV disinfection.
9. UV-Visible Absorption Spectroscopy:
UV-visible absorption spectroscopy is a versatile technique used to analyze the absorption of light by a sample across a range of UV and visible wavelengths. This method provides valuable information about the electronic structure and chemical composition of the sample.
UV-visible spectrophotometers employ a light source that emits UV and visible radiation, a monochromator to select specific wavelengths, a sample holder, and a detector to measure the intensity of transmitted light. By recording the absorption spectrum of a sample, researchers can identify absorbing species and quantify their concentrations.
10. UV Chromatography:
UV chromatography involves coupling UV detection with chromatographic separation techniques to analyze UV-absorbing compounds in complex mixtures. High-performance liquid chromatography (HPLC) and gas chromatography (GC) are commonly used chromatographic methods that can be equipped with UV detectors.
UV detectors in chromatography systems measure the absorbance of eluting compounds at specific UV wavelengths as they pass through a flow cell. By correlating the UV absorbance with the concentration of analytes, chromatography coupled with UV detection enables quantitative analysis of UV-active compounds in samples ranging from pharmaceuticals to environmental samples.
Safety Precautions for UV Light Measurement
Measuring UV light, particularly in the context of high-intensity sources, requires careful attention to safety precautions to protect both the operator and the equipment. Prolonged or intense exposure to UV radiation can cause harm to the eyes and skin, including burns and increased risk of cancer.

To mitigate these risks, operators should wear protective eyewear that blocks UV wavelengths and cover skin with clothing or special protective materials. It’s also crucial to limit exposure time and use UV shielding or barriers around high-intensity UV sources whenever possible. Additionally, ensuring that UV measurement instruments are correctly calibrated and maintained can help prevent accidental overexposure due to incorrect readings.
Laboratories and workplaces should also adhere to local regulations and guidelines regarding UV radiation safety, implementing procedures for safe operation and emergency response in the event of UV exposure incidents.
Interpreting UV Light Measurement Data
Interpreting UV light measurement data is a critical step in understanding the effects of UV radiation in various applications. The data acquired from UV measurement instruments can provide insights into the intensity, wavelength distribution, and potential biological or chemical effects of UV radiation.
Accurate interpretation requires a strong understanding of the type of UV radiation being measured (UVA, UVB, or UVC) and its relevance to the specific application at hand. For instance, in phototherapy, the therapeutic dose depends on the precise measurement of UVB radiation, while in UV disinfection, the effectiveness is linked to the dose of UVC radiation.
Data analysis often involves comparing measured UV intensities against known standards or guidelines to assess compliance or efficacy. Additionally, it’s essential to consider the spectral response of the detector and the calibration of the instrument when analyzing the data.
Common Mistakes to Avoid When Measuring UV Light
Measuring UV light accurately is crucial for various scientific, industrial, and health-related applications. However, common mistakes can lead to inaccurate measurements and potentially harmful consequences. One of the primary errors involves using uncalibrated or improperly calibrated equipment. Calibration ensures that instruments provide accurate readings; thus, neglecting this step can result in misleading data.

Another mistake is ignoring the spectral sensitivity of the sensors. Different sensors have different responses to various UV wavelengths, and not accounting for this can skew results. Additionally, overlooking environmental factors, such as ambient light and temperature, can also affect UV light measurements. These factors can either absorb or reflect UV radiation, altering the accuracy of the readings.
Conclusion
In conclusion, measuring UV light is essential across various industries and scientific fields due to its significant implications for health, safety, and environmental monitoring. By employing sophisticated instruments such as UV meters, radiometers, and spectrophotometers, researchers, engineers, and health professionals can accurately quantify UV radiation levels.
Understanding the properties of UV light, selecting appropriate measurement methods, and considering factors like calibration and safety precautions are vital for obtaining precise data. With advancements in technology and increased awareness of UV light’s effects, the demand for reliable UV measurement tools continues to grow. Thanks for reading, and we hope this has given you some inspiration on how to measure uv light!

